- AdventHealth
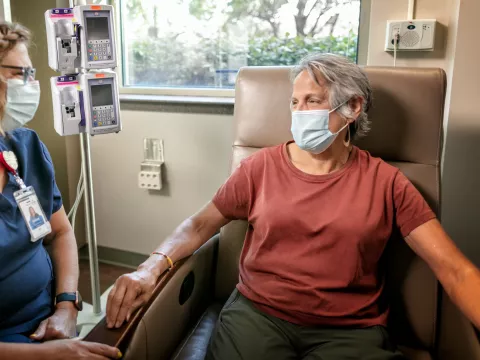
Those at high risk for severe COVID-19 now have access to a potentially lifesaving antibody treatment that can reduce the likelihood of becoming seriously ill from COVID-19. This treatment, offered at AdventHealth’s locations across the country, is the latest in a suite of COVID-19 therapeutics aimed at helping those with COVID-19 recover safely.
A single dose of monoclonal antibodies bamlanivimab and etesevimab administered together has been found to lower the chance of hospitalization or ER visits for people with mild-to-moderate COVID-19 who are at greater risk of hospitalization due to their age or a chronic medical condition, according to the FDA.*
Monoclonal antibody combinations, which are secured by the federal government and then distributed to states, contain proteins that mimic the immune system’s ability to fight the virus. The monoclonal antibody combinations that are FDA emergency use authorized include bamlanivimab and etesevimab, as well as casirivimab and imdevimab, commonly known as Regeneron.*
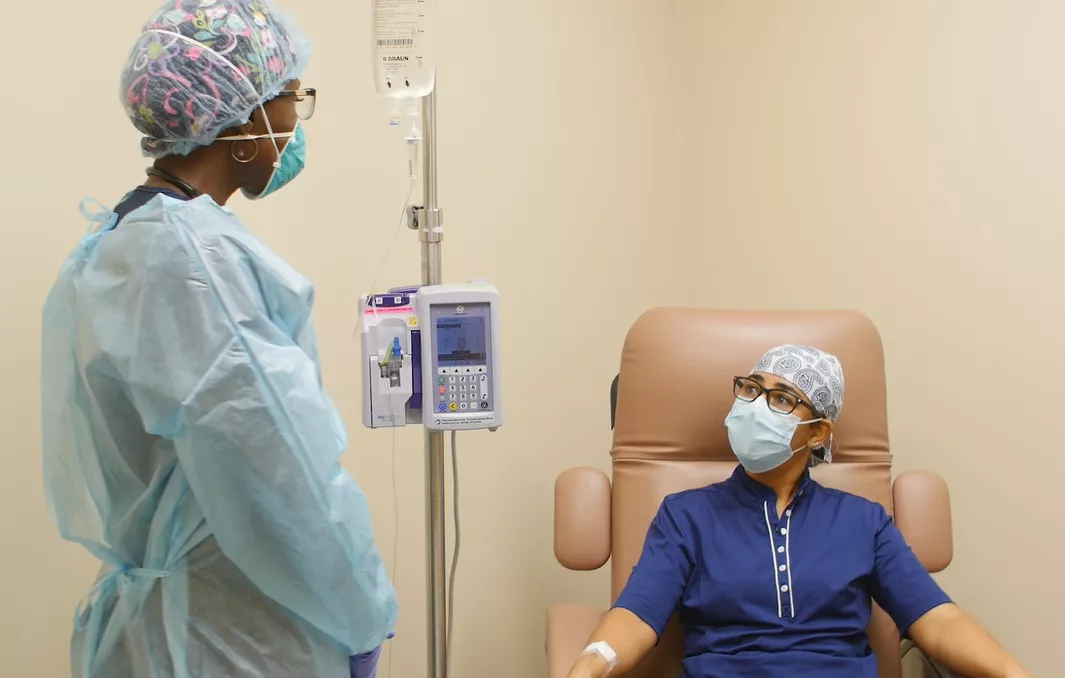
Doctors are able to prescribe the treatment to patients with an onset of symptoms within the past 10 days who have high-risk factors such as diabetes, heart disease or obesity and are not hospitalized or on oxygen. Some AdventHealth locations have opened dedicated antibody treatment clinics where patients can comfortably receive the hour-long intravenous infusion.
“We are thrilled to offer this lifesaving treatment to those at high risk for severe COVID-19 as a way to reduce hospitalizations and allow safe recoveries from the comfort of their own home,” said Brent Box, MD, chief medical officer for AdventHealth.
Monoclonal antibody treatments join a host of other COVID-19 therapies offered at AdventHealth including remdesivir, sarilumab, dexamethasone and convalescent plasma. The health system hopes to continue increasing its capacity for administering this treatment as federal supply distribution allows.
*This article was updated based on new information from the FDA, released on April 16, 2021.
Recent News

AdventHealth South Overland Park awarded Primary Stroke Center Certification from The Joint Commission
AdventHealth South Overland Park has earned The Joint Commission’s Gold Seal of Approval® and the American Heart and Stroke Association’s Heart-Check mark for Primary Stroke Certification.

Former patient returns to AdventHealth Littleton to comfort moms in the NICU
A former patient of AdventHealth Littleton returned to recognize NICU moms on Mother’s Day.

Protecting Your Skin: Insights and Innovations During Skin Cancer Awareness Month
Skin Cancer Awareness Month serves as a crucial reminder of the importance of vigilance in detecting and preventing skin cancer, the most common cancer affecting both men and women in the United...

Rob Deininger named president/CEO of AdventHealth’s East Florida Division
AdventHealth has named Rob Deininger president and CEO of its East Florida Division, which includes facilities in Volusia, Lake and Flagler counties, effective May 4, 2025.

Celebrating EMS Week: Honoring our EMS teams in the Rocky Mountain Region
Emergency Medical Services (EMS) Week is a time to honor the incredible dedication and exceptional skill of EMS providers who operate tirelessly to safeguard our communities.

Mission Control: 5 years of smarter, faster patient care
AdventHealth’s Mission Control, launched five years ago, serves as a central hub using real-time data and AI to manage patient care across Central Florida.
